How is iAluRil administered?
iAluRil is administered intravesically as a 50ml bladder instillation via a catheter (with Luer-Lock adapter) or using the iAluadapter®.
Self administration of iAluRil is permitted following appropriate training.
Administration using a catheter
- After the patient has urinated spontaneously, empty the bladder of all traces of urine by inserting a suitable sterile catheter through the external urethral meatus (use of an 8Ch catheter is recommended during this stage).
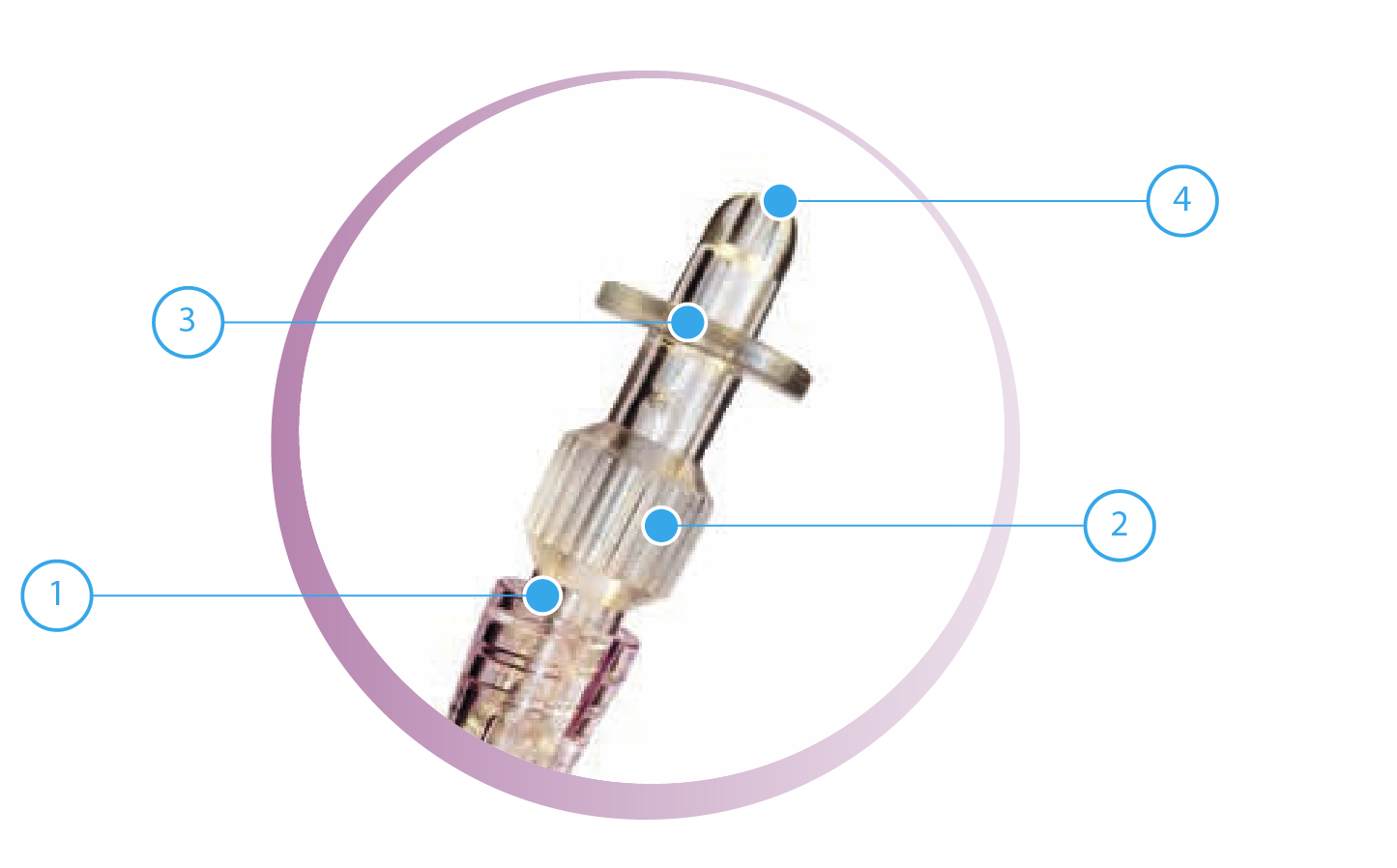
- Screw the plunger rod supplied with the prefilled syringe, until it is perfectly in place.
- Mount the Luer-Lock adapter on the top of the prefilled syringe and apply onto it the sterile catheter previously placed in the bladder.
- Instil all the solution contained in the syringe into the bladder through the catheter.
- Keep iAluRil in the bladder for between 30 minutes and 3 hours.
Administration using the iAluadapter
- Ask the patient to completely empty their bladder.
- Attach the iAluadapter onto the iAluRil pre-filled syringe using a no touch technique and a half-twist motion.
- Lubricate the tip by squeezing a small amount of iAluRil from the syringe to just sit on the tip of the iAluadapter.
- Insert the tip of the iAluadapter into the urethra; rotating to ensure a good fit.
- Enhance pressure on the syringe to slowly instil, reminding the patient to relax as much as possible.
- Keep iAluRil in the bladder for between 30 minutes and 3 hours.