Bladder Pain Syndrome/Interstitial Cystitis (BPS/IC)
Reference: Cervigni M. et al 2008 ‘…seems to offer an additional therapeutic option in patients with refractory PBS/IC.’
Method
In this study, 23 women with IC/PBS, who had failed several previous oral treatments and intravesical instillations of hyaluronic acid (HA) 0.08%, were administered iAluRil weekly for 20 weeks then every 2 weeks for 4 weeks and then monthly for 3 months. Of the 23 patients who entered the study, there was no dropout of patients during the treatment period and all completed the protocol. No cases of intolerance, side effects or complications were observed.
Patients were evaluated before treatment, monthly during treatment and at 5 months follow up using a range of patient questionnaires (i.e. O’Leary-Sant Interstitial Cystitis Symptom Index [ICSI] and Problem Index [ICPI], Pelvic Pain and Urgency/Frequency Patient Symptom Scale and Visual Analogue Scale for pain, frequency and urgency), complete urinalysis and urine culture, a 3-day voiding diary and a complete urodynamic study.
Results
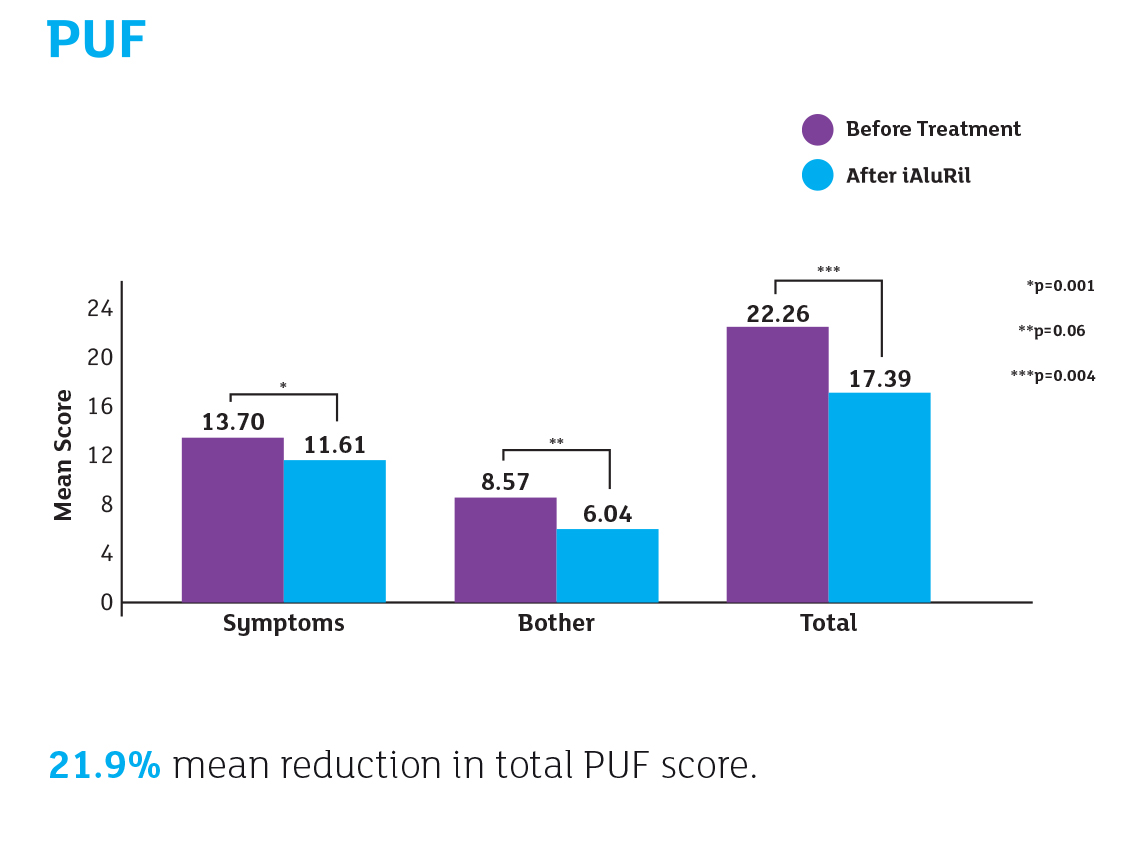
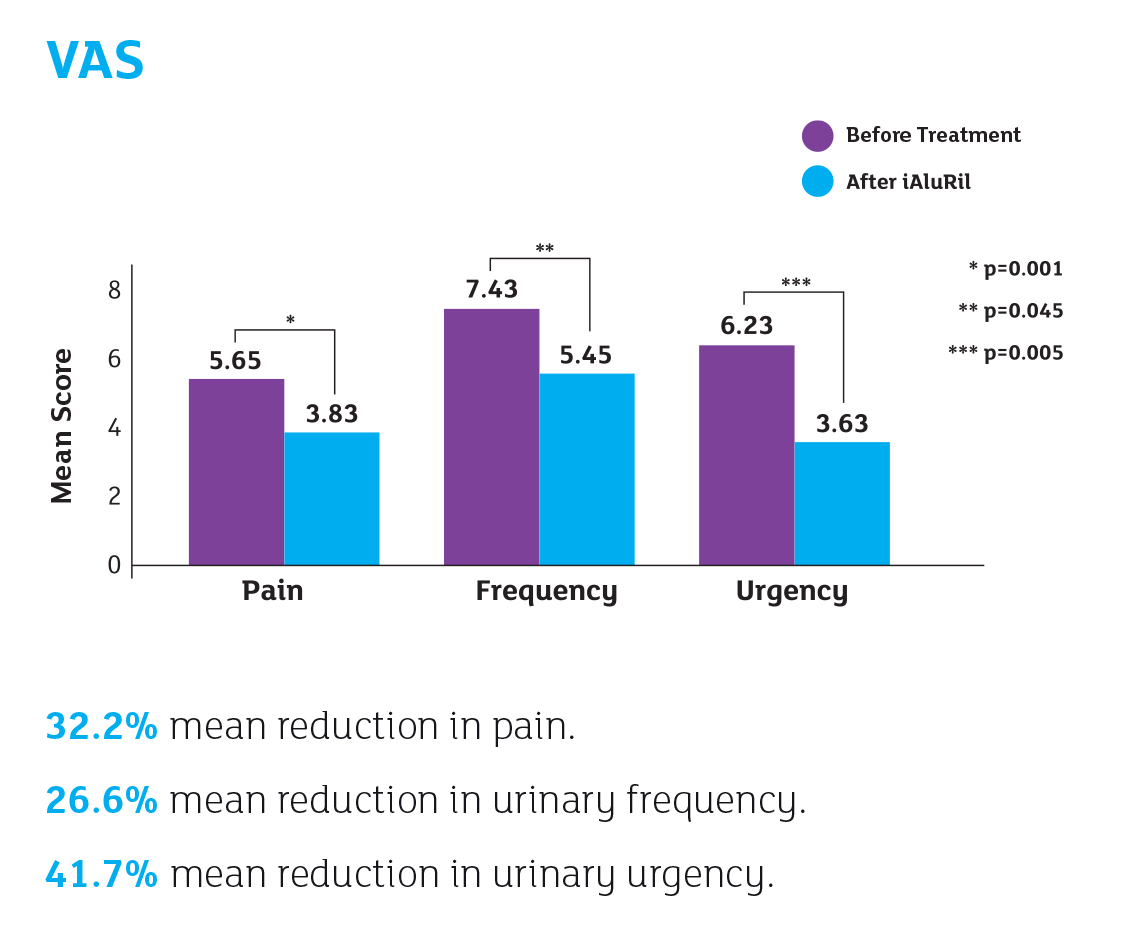
Reference
Cervigni M et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/ interstitial cystitis.
Int. Urogynecol J. 19, 943-947 (2008).
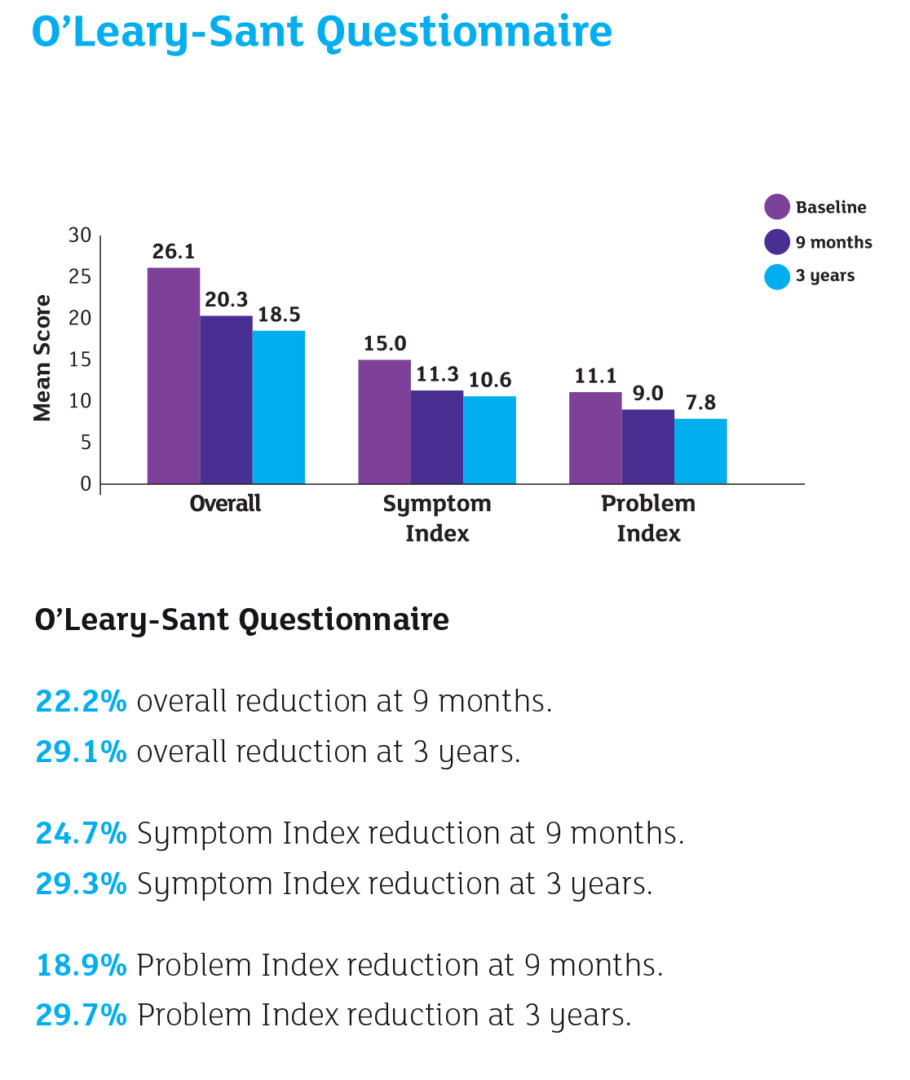
Reference: Cervigni M. et al 2012 ‘Intravesical instillations of HA 1.6 % and CS 2.0 % over a period of 9 months produced a sustained improvement in symptoms, which was still apparent at 3-year follow-up, in patients affected by PBS/IC unresponsive to previous treatments’
Method
12 women (34 – 65 years (mean 52.6 years)) were enrolled in the study. All patients had failed previous treatments: pentosan polysulfate, cimetidine and dimethyl sulphoxide (DMSO); 2 patients had also undergone intradetrusorial administration of botulinum toxin. No other alternative or interim treatments were prescribed during the 3 year follow-up period.
- Patients were administered iAluRil weekly for 20 weeks, then every 2 weeks for 4 weeks and then monthly for 3 years.
- Follow-up visits were conducted at 9 months and 3 years.
- Follow-up evaluations consisted of 3-day voiding diary, VAS and validated questionnaires (O’Leary-Sant Questionnaire and PUF).
Results
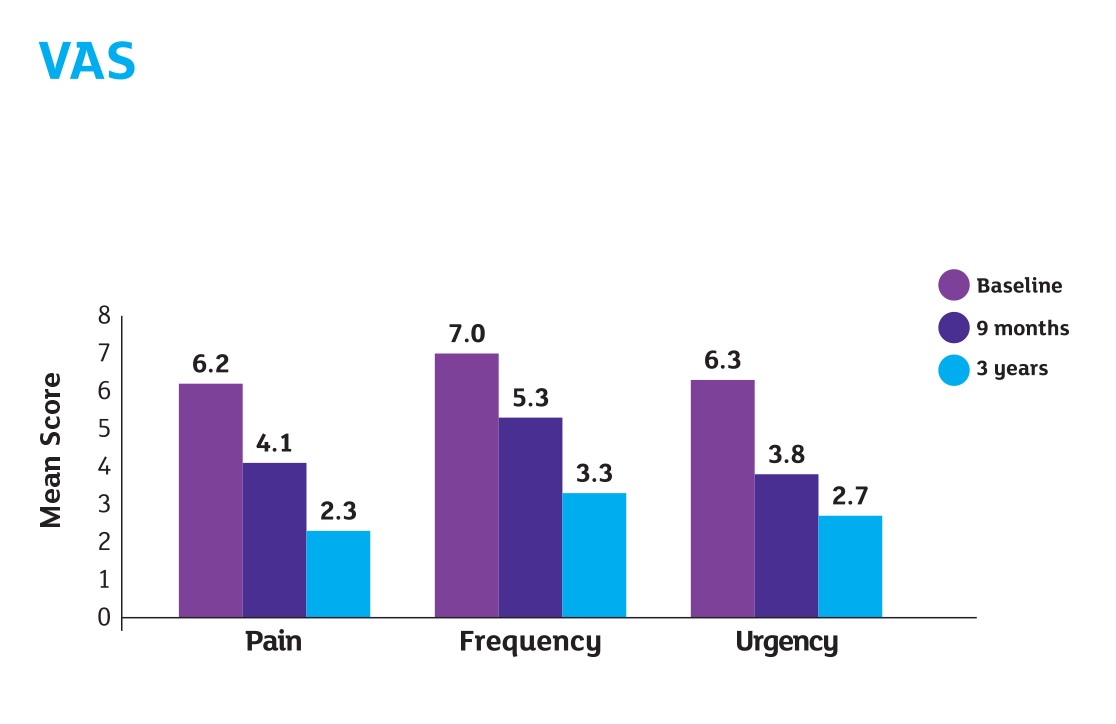
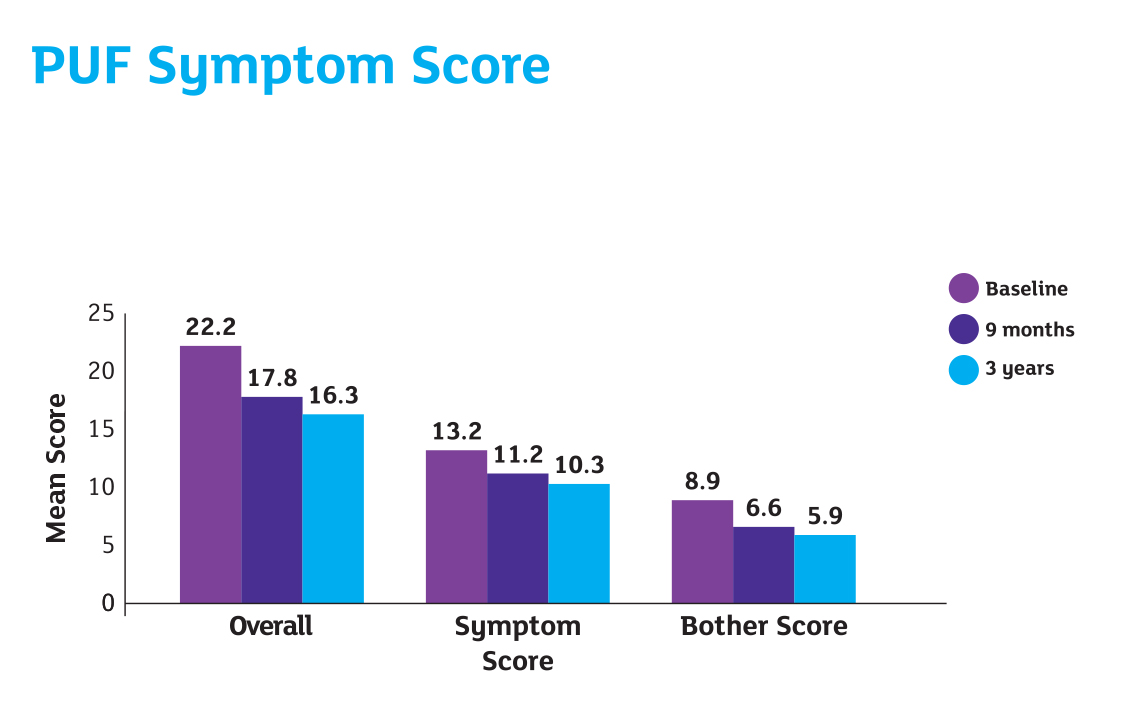
Reference
Cervigni M et al. Intravesical Hyaluronic Acid and Chondroitin Sulfate for Bladder Pain Syndrome/Interstitial Cystitis:
Long-term Treatment Results. Int Urogynaecol J 2012; 23(9): 1187-1192.
Reference: Cervigni M. et al 2016 ‘Treatment with HA/CS appears to be as effective as DMSO with a potentially more favourable safety profile. Both treatments increased health related quality of life, while HA/CS showed a more acceptable cost-effectiveness profile.’
Method
This study compared the efficacy and safety of intravesical HA/CS (iAluRil) to dimethyl sulfoxide (DMSO) in 110 women with BPS/IC (mean age 50.24), where patients experienced pain and at least one other urinary symptom (i.e., urgency or frequency) for ≥6 months.
Patients were randomised 2:1 to iAluRil or DMSO. 74 patients were treated with iAluRil and 36 patients were treated with DMSO. Patients received weekly instillations for 13 weeks.
At 6 months, 59 patients for iAluRil and 31 patients for DMSO completed the follow-up.
Results
During treatment phase, 3 iAluRil patients and 4 DMSO patients discontinued due to lack of efficacy. 1 patient from each group withdrew from the study due to treatment-related adverse events. At 6 months, 59 patients for iAluRil and 31 patients for DMSO completed the follow-up.
Adverse events were experienced in 30.56% of patients treated with DMSO and 14.86% of patients treated with iAluRil. VAS pain reduction was significant in both groups.
Reference
Cervigni M et al. A randomized, open-label, multicenter study of the efficacy and safety of intravesical hyaluronic acid (HA) and chondroitin sulfate (CS) versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourology and urodynamics;36(4):1178-1186. doi: 10.1002/nau.23091. Epub 2016 Sep 21.
Reference: Cocci A. et al 2016 ‘All three instillations were effective in BPS/IC patients but iAluRil was the most effective…and it was the simplest for patients to self-administer”. In terms of cost-effectiveness we would recommend that patients are started on the Whitmore cocktail for 6 weeks and if not successful then they are switched to iAluRil’
Method
A pragmatic retrospective analysis from January 2010 to September 2015 in a tertiary referral centre to compare Cystistat (50ml sterile sodium hyaluronic acid) (n = 145), iAluRil (n = 54), and Whitmore Cocktail (bupivacaine 0.5% x40ml, hydrocortisone 100 mg, heparin 10,000 units, sodium chloride 0.9% x 1Oml) (n=50) for treatment of patients with bladder pain syndrome/interstitial cystitis (BPS/IC).
All patients had the same instillation protocol: weekly for 6 weeks, then fortnightly for 4 weeks, then monthly for 6 months.
Patient satisfaction regarding self-administration, relief of symptoms, switch between instillations, changes in frequency of instillation were analysed.
Results
100% of iAluRil patients did not need to switch to alternative treatments whereas 21% of patients on Cystistat and 22% of patients on Whitmore switched to iAluRil.
Only 7.5% of patients on iAluRil stayed on monthly instillations whereas 9% of patients started on Cystistat and 18% of patients started on Whitmore Cocktail stayed on monthly instillations.
Reference
Cocci A. et al. Comparison of Cystistat, iAluRil, and Whitmore Cocktail for treatment of patients with bladder pain syndrome/interstitial cystitis. Poster presentation. EAU16, Munich, Germany, 11-15 March 2016.
Recurrent Urinary Tract Infection (rUTI)
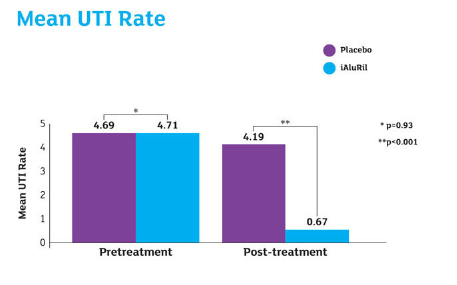
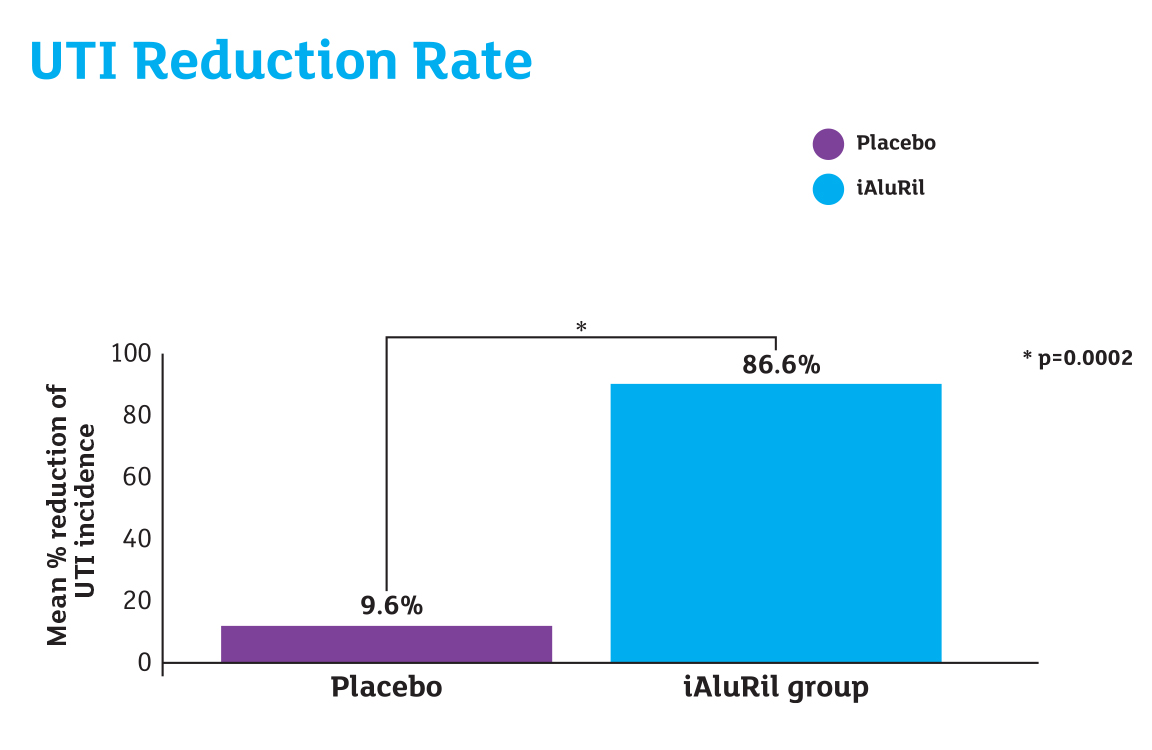
Reference: Damiano R. et al 2011 ‘…iAluRil intravesical instillations significantly reduced UTI rate without severe side effects while improving symptoms and QoL over a 12-month period in patients with rUTI.’
Method
Patients (57 women (mean age: 34.8 years) with 3 or more UTIs (>10³ CFU/ml) within previous year) were randomised 1:1 to iAluRil (n = 28) or placebo (saline; n = 29); 3 patients were lost to follow-up (n=27 for both treatment arms). They then received treatment or placebo weekly for four weeks followed by monthly for 5 months.
The instillations were left in the bladder for at least 2 hours before voiding. No prophylactic antibiotics were given before, during or after instillations. Urinalysis and urine culture were carried out 3 days prior to instillations to check urine sterility.
Follow-ups were carried out at month 1, month 3, month 6, month 9 and month 12. Patients were assessed for UTI status (midstream specimen of urine), storage and voiding.
Patients also completed SF-36 QoL questionnaires at each follow-up.
Results
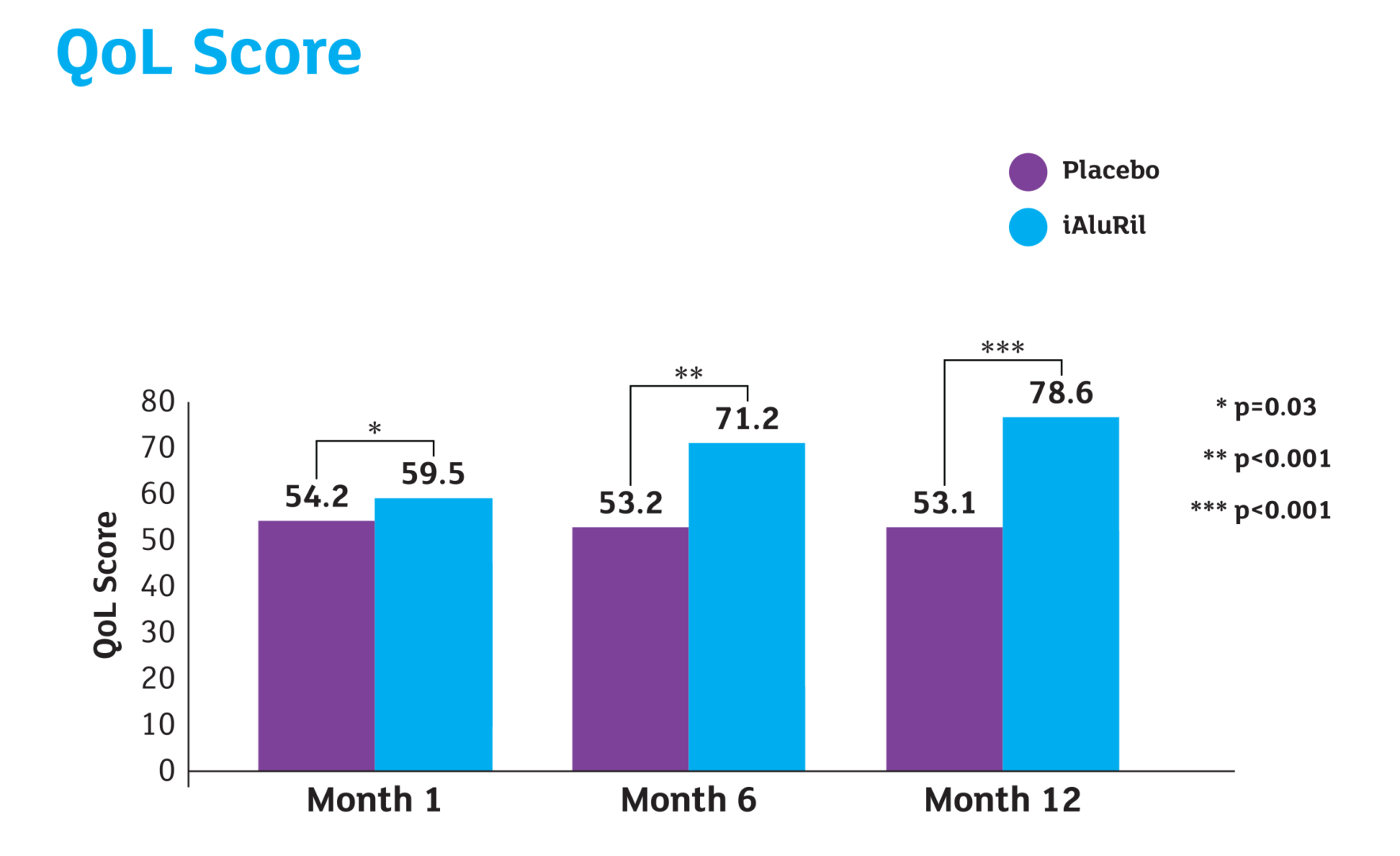
Mean QoL Score:
Subjective Analysis (QoL) Scores
At month 1: Placebo 54.2 Vs iAluRil 59.5 (p=0.03)
At month 3: Placebo 52.5 Vs iAluRil 59.7 (p=0.005)
At month 6: Placebo 53.2 Vs iAluRil 71.2 (p<0.001)
At month 9: Placebo 53.1 Vs iAluRil 71.1 (p<0.001)
At month 12: Placebo 53.1 Vs iAluRil 78.6 (p<0.001)
Reference
Damiano R et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur. Urol. 59(4), 645-651 (2011).
Reference: Cicione A. et al 2014 ‘…intravesical HA-CS administration is an effective and well tolerated non-antibiotic treatment option in women with rUTI.’
Method
A retrospective cohort multicentre study involving 7 European centres. 157 women (mean age 54.2 ± 4.1 years) with documented history of at least 3 episodes of uncomplicated UTIs with the isolation of >103 CFU/mL of an identified pathogen with clinical symptoms in the last 12 months were studied.
Patients received iAluRil weekly for 4 weeks then monthly for 5 months (a total of 9 instillations). 28% of patients received a different regimen due to further instillations up to 12 months. Instillations for positive urine culture was delayed in 23 cases (14.6%).
Following baseline data capture, patients were assessed at 1 month, 6 months and 12 months (18-month and 24-month assessments were also carried out but for the purpose of this summary have not been used as patient numbers reduced and treatment had stopped on/before 12 months).
At every visit patients completed: Pelvic Pain and Urgency/Frequency Symptom Scale (PUF) and SF-36 QoL questionnaire. Urinalysis and urine culture were performed before each instillation and at each follow-up visit.
Results
At 12 months:
- 89.35% reduction in mean UTI rate per person from 4.13 to 0.44 (p=0.01)
- 28.32% reduction in mean total PUF score from 20.09 to 14.4 (p=0.01)
- 29.93% reduction in mean total symptom score from 13.7 to 9.6 (p=0.01)
- 31.51% increase in mean QoL SF-36 score from 59.25 to 77.92 (p=0.01)
Reference
Cicione A et al. Intravesical treatment with highly-concentrated hyaluronic acid and chondroitin sulphate in patients with recurrent urinary tract infections: Results from a multicentre study. Can Urol Assoc J. 2014; 8(9-10): E721-7
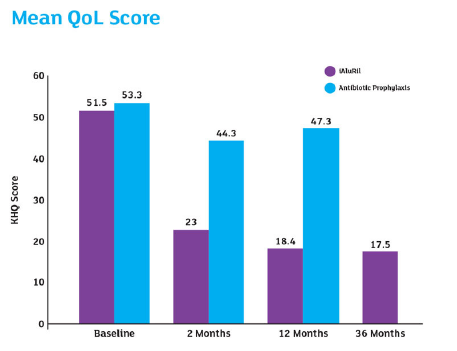
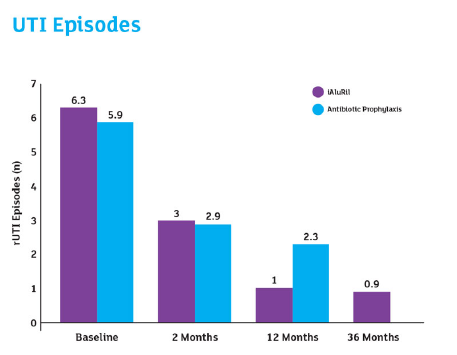
Reference: De Vita et al 2012 & 2018 ‘…iIntravesical instillation of hyaluronic acid/ chondroitin sulfate is an effective and well-tolerated non- antimicrobial treatment for patients with recurrent bacterial cystitis that provides sustained clinical benefits for up to 36 months after treatment.’
Method
28 women (60 ± 13 years) with rUTI in the previous 12 months were randomised to iAluRil weekly for 4 weeks, fortnightly for 1 month (n=14) or long-term antibiotic prophylaxis sulfamethoxazole 200mg and trimethoprim 40mg, once weekly for 6 weeks (n=14). 26 completed the follow-up at 12 months as 2 iAluRil patients were lost to follow-up.
Results
Patients treated with iAluRil showed a reduction in the number of cystitis (UTI) recurrences, significantly reduced at 12-month follow-up (p=0.02).
The long-term effects of iAluRil at 36 months after treatment (n=12) were then evaluated.
Reference
De Vita D et al. Effectiveness of Intravesical Hyaluronic Acid/Chondroitin Sulfate in Recurrent Bacterial Cystitis: a Randomized Study. Int Urogynecol J 2012; 23(12): 1707-1713. De Vita D, et al. Long-term efficacy of intravesical instillation of hyaluronic acid/ chondroitin sulfate in recurrent bacterial cystitis: 36 months’ follow-up. Clin. and Exp. Obstet. & Gynecol. 2018 – CEOG XLV n.2
Reference: Torella. M et al 2013 ‘It is evident that the local administration of GAGs, in particular HA and CS, in the bladder is a critical factor in reducing urinary recurrent infections.’
Method
The aim of this study was to assess whether intravesical therapy with HA and CS (iAluRil) is more effective than antibiotic therapy in reducing episodes and symptoms of rUTIs.
69 Patients were enrolled from 3 different centres (average age 58.6). Each patient had a minimum of 2 uncomplicated UTIs in the previous 6 months or 3 uncomplicated UTIs in the previous 12 months.
No patients had active UTIs. Patients were assigned to three groups (group A: fosfomycin (n=23), B: iAluRil (n=22) or C: fosfomycin + iAluRil (n=24)).
Results
Patients treated with iAluRil showed a reduction in the number of cystitis (UTI) recurrences, significantly reduced at 12-month follow-up (p=0.02).

Reference
Reference: Torella M et al. Intravesical Therapy in Recurrent Cystitis: a Multi-Center Experience. J Infect Chemother 2013; 19(5): 920-925
Chemical Induced Cystitis
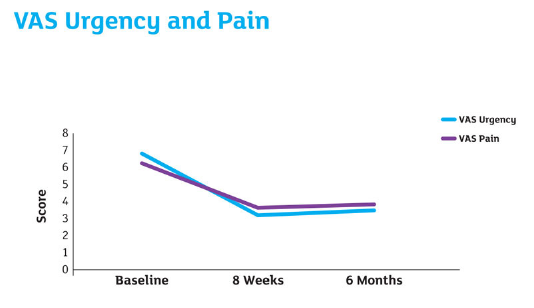
Reference: Creta M. et al 2012 ‘Intravesical therapy with HA and CS provides a sustained improvement of urgency, pain, frequency and bladder capacity in patients with BCG-induced cystitis.’
Method
15 patients with grade 2 (>48 hours) chemical cystitis following intravesical instillation of Bacillus Calmette-Guerin (BCG) and unresponsive to traditional therapy were treated with intravesical instillation of iAluRil weekly for 8 weeks. Outcome measures were mean voiding volume, urinary frequency, urgency and bladder pain, which were measured prior to treatment, at 8 weeks and at 6 months follow up. This was done using a voiding diary and VAS with values ranging from 0-10 for urgency and pain.
Results
After 8 weeks of weekly treatment:
- 51.5% reduction in VAS urgency
- 42.2% reduction in VAS pain
- 37.4% reduction in mean voids per 24 hours
- 88.3% increase in mean urine volume per void.
VAS pain – baseline: 6.4, 8 weeks: 3.7, 6 months: 3.8
VAS urgency – baseline: 6.8, 8 weeks 3.3 and 6 months 3.5
Reference
Creta M et al. Intravesical instillation of hyaluronic acid and chondroitin sulfate for bacillus calmette-guerin induced chemical cystitis. Poster presentation P164, 85th SIU (Italian Society of Urology) 2012; October 21-24
Reference: Imperatore V. et al 2018 ‘...intravesical instillation of iAluRil was well tolerated by all patients, with no side effects observed during treatment, and is an efficacious treatment for refractory BCG-induced chemical cystitis.’
Method
The charts of 20 patients (9 males and 11 females) with Bacillus Calmette-Guérin (BCG)-induced grade 2 (severe and/or > 48-hour cystitis according to the World Health Organisation [WHO]) chemical cystitis, who had failed at least 1 month of conventional therapies (quinolone antibiotics and anti-inflammatory agents), were reviewed.
Following suspension of BCG instillations, patients were treated weekly with iAluRil for 8 weeks. Patients’ charts were reviewed retrospectively.
Data was collected at baseline which included demographics, patient recorded VAS score (1-10) for bladder pain and urgency, and functional bladder capacity and daytime urinary frequency which was measured using 3-day frequency/volume charts.
Follow-up data was collected at 8 weeks, 6 months and 1 year using a 3-day voiding diary and patient recorded VAS scores for urinary urgency and pain.
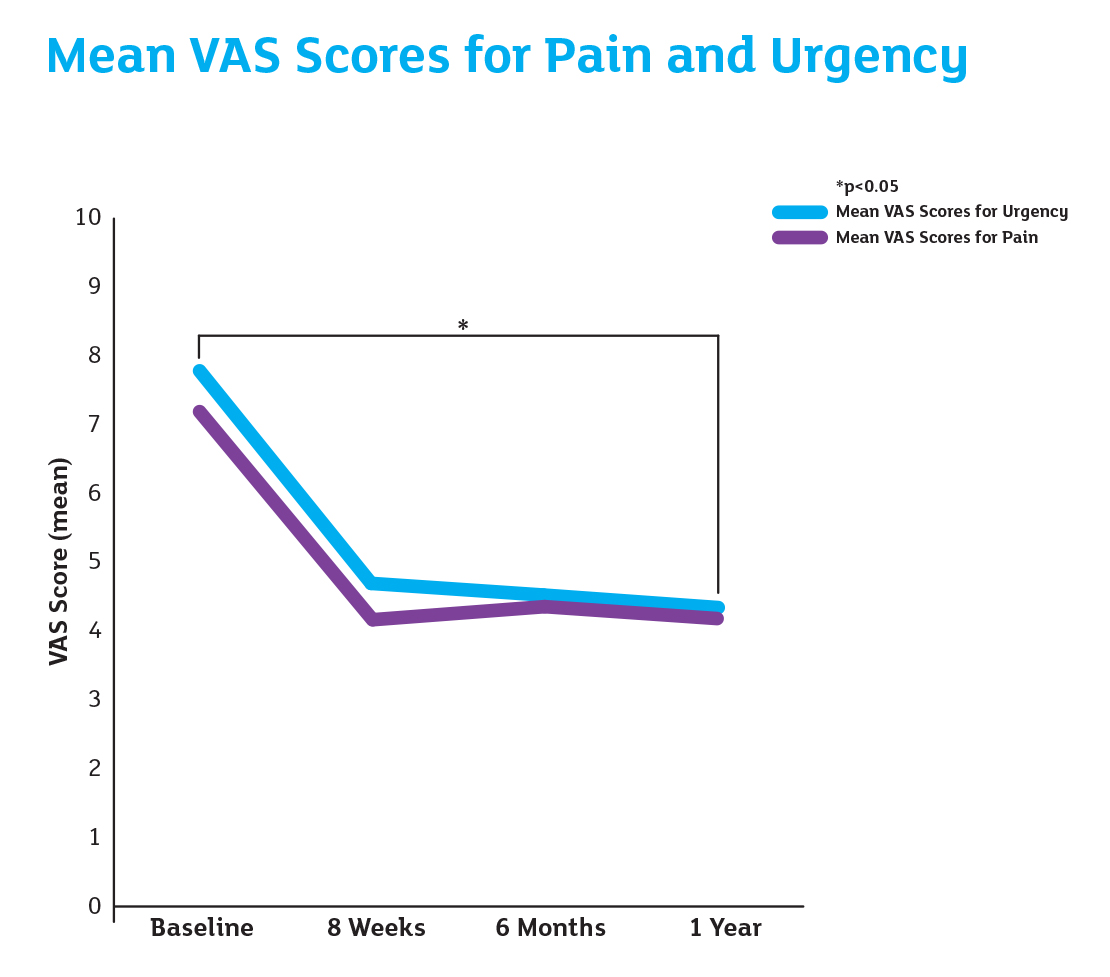
Reference
Imperatore V et al, Intravesical administration of combined hyaluronic acid and chondroitin sulfate can improve symptoms in patients with refractory bacillus Calmette-Guerin-induced chemical cystitis: Preliminary experience with one-year follow-up. Arch Ital Urol Androl. 2018 Mar 31; 90(1): 11-14
Radiation Induced Cystitis
Reference: Gacci M. et al 2016 ‘...bladder instillation with HA and CS is a safe treatment in post radiation bladder cystitis. Patients after Ialuril® instillation reported a statistically significant reduction in symptom/bother nocturnal voiding frequency...’
Method
The efficacy and safety of hyaluronic acid and chondroitin sulfate (HA-CS) instillation in 80 consecutive men treated with RT for prostate cancer (PCa) as primary or adjuvant treatment was evaluated. 30 (37.5%) of these patients reported clinically relevant lower urinary tract symptoms (LUTS) and were enrolled in the study (mean age: 69.1 years).
Following RT, the 30 patients who reported clinically relevant LUTS and associated bother (ICSI and ICPI ≥3) were entered into the study. 10 men had received primary RT for PCa and 20 men had received adjuvant RT for PCa. 3 (10%) patients had low-risk PCa, 13 (43%) had intermediate-risk PCa and 14 (47%) had high-risk PCa.
The mean radiation dose was 67 Gy (range 62-80 Gy). All patients reported ≥ grade 1 toxicity according to the Radiation Oncology Group. Patients were treated with iAluRil, weekly for 4 weeks, then at weeks 6, 8 and 12.
Results
HA-CS instillation significantly reduced postradiation LUTS (P < 0.001) and bother (P = 0.006) irrespective of age and clinical features, with full recovery of urinary bother.
Reference
Gacci M et al. Bladder Instillation Therapy With Hyaluronic Acid and Chondroitin Sulfate Improves Symptoms of Post radiation Cystitis: Prospective Pilot Study. Clin Genitourin Cancer. Oct;14(5):444-449 (2016)
Reference: Gianessi C. et al 2014 ‘iAluRil was effective in reducing the symptoms and associated bother of nocturia in patients with post radiation BPS. However, further randomized controlled trials are required to confirm the results of this study.’
Method
23 male patients were studied, mean age 67.9, with BPS due to pelvic irradiation for locally advanced PCa (16 patients underwent radical prostatectomy and RT, and 7 underwent RT alone).
Patients underwent intravesical administration of iAluRil weekly for the first month, and on the 6th, 8th and 12th week subsequently.
Nocturia was assessed immediately after RT and at 12 weeks after the final iAluRil instillation using item 3 (Q3) of the ICSI which asks how often patients experience nocturia and item 2 (Q2) of the ICPI which asks how much of a problem the patient finds nocturia.
Results
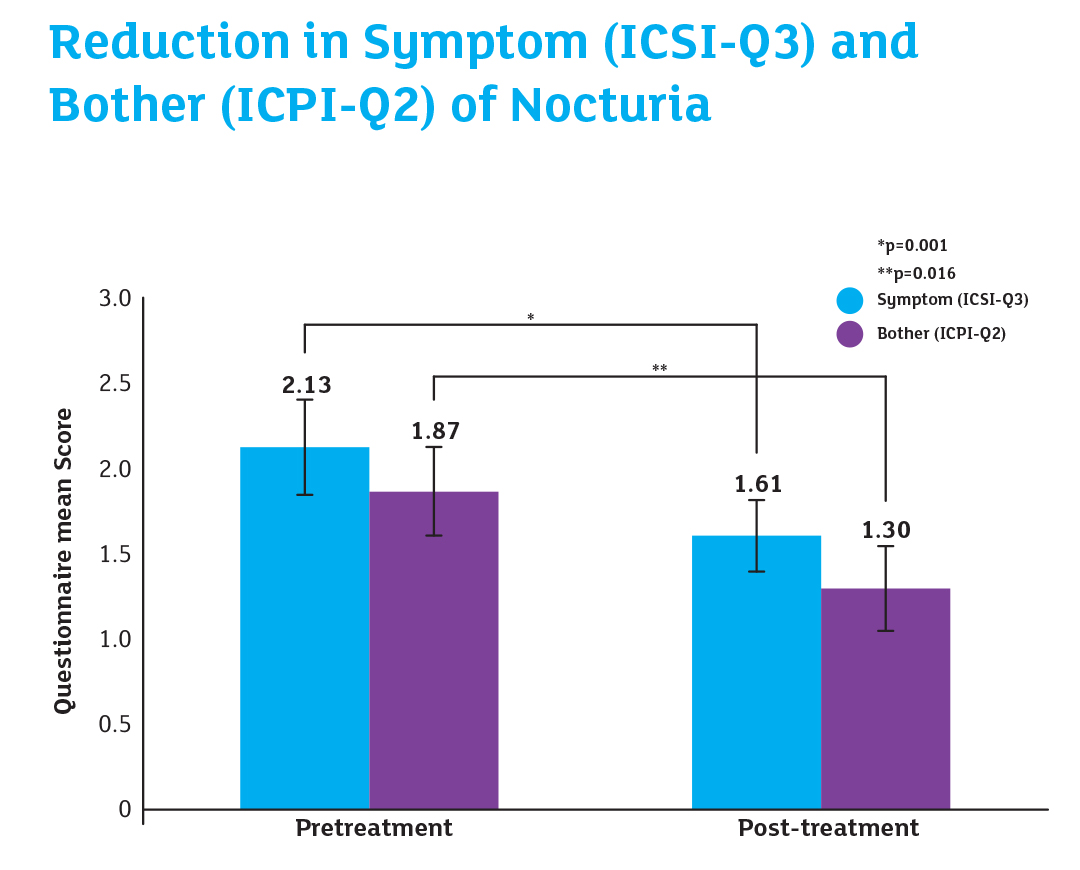
Reference
Giannessi C et al. Nocturia related to post bladder pain can be improved by hyaluronic acid chondroitin sulphate. Eur Urol Suppl. 2014; 13; e592
Ureteric Stent
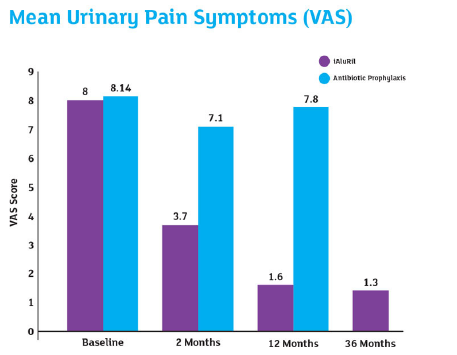
Reference: Jung Ki Jo et al 2018 ‘Highly concentrated HA/CS instillation improved ureteric stent-induced urinary symptoms. It also significantly reduced pain, at least in the short term. Moreover, it reduced the need for additional medication or procedures after stent placement.’
Method
92 patients (49 men and 43 women) completed the study, 46 and 46 were in the treatment and control arms, respectively. Eligible patients, who received an indwelling ureteric stent after ureteroscopic lithotripsy for a first-time ureteric stone, were randomly allocated to receive intravesical instillation with highly concentrated hyaluronic acid (HA)/chondroitin sulphate (CS) or normal saline just after ureteric stent placement.
The HA/CS instillation was prophylactic for relief of ureteric stent-related symptoms*.
Just before stent removal on postoperative day 7, the patients completed the Ureteric Stent Symptom Questionnaire (USSQ), International Prostate Symptom Score (IPSS) QoL question, and a pain visual analogue scale (VAS).
Results
Mean Urinary Pain Symptoms: P<0.001
IPSS Q0L Score: P=0.018
USSQ Score, mean: P<0.001
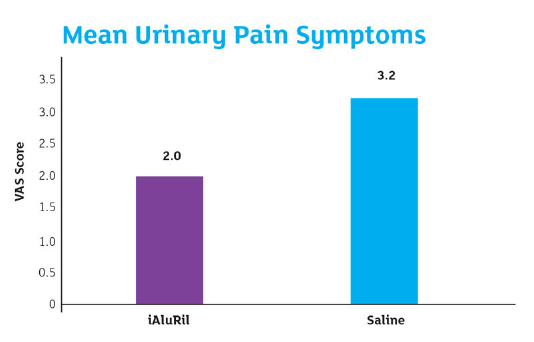
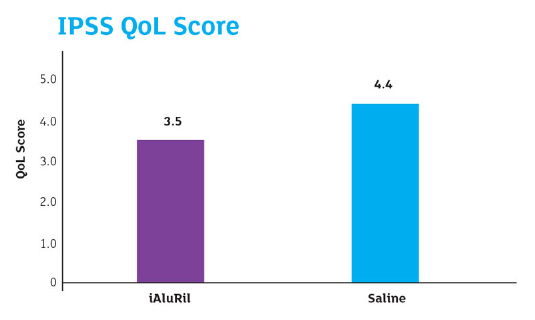
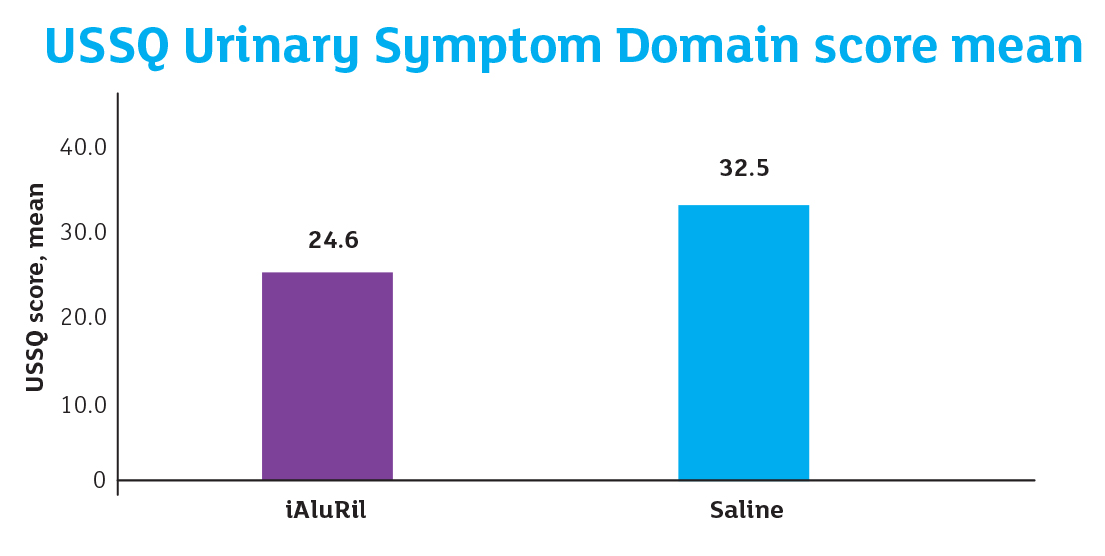
Reference
Jo JK et al. Effect of highly concentrated hyaluronic acid/chondroitin sulphate instillation on ureteric stent-induced discomfort after ureteroscopic lithotripsy: a multicentre randomised controlled pilot study. BJU Int. 2018 May 17.
*This study meets the current product indication, regarding restoration of the bladder GAG layer. However, the posology for the treatment was not respected.